عنوان
304 شارع الكاردينال الشمالي
مركز دورتشستر ، ماساتشوستس 02124
ساعات العمل
من الاثنين إلى الجمعة: 7 صباحًا - 7 مساءً
عطلة نهاية الأسبوع: 10 صباحًا - 5 مساءً
مجموعة شاندونغ الخبراء للمعدات الطبية
تصر مجموعة Expert Medical Equipment Group ، باعتبارها واحدة من أكثر الشركات المصنعة والمصدرة للمعدات الطبية احترافًا في الصين ، على توفير منتجات عالية الجودة والسلامة والموثوقية بالإضافة إلى أفضل خدمات ما بعد البيع للسوق العالمي.


سلسلة جداول العمليات الطبية الخبيرة تلبي متطلبات الجراحة العامة والقلب والرأس والرقبة وتجويف الصدر وغيرها من العمليات الجراحية.
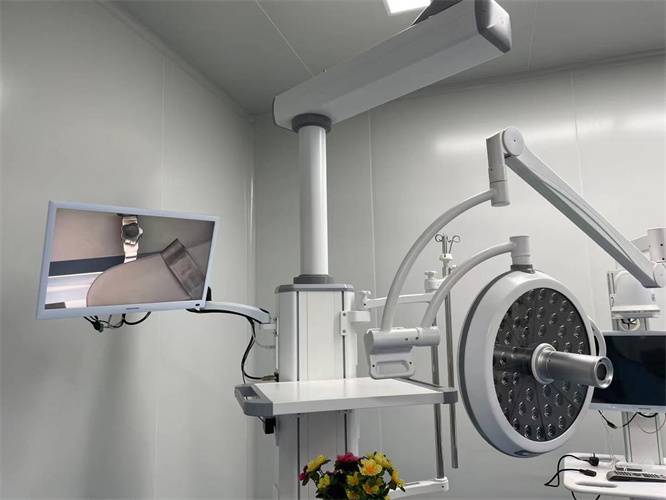
سلسلة مصابيح التشغيل الطبية الخبيرة تشارك في مختلف مجالات الإضاءة الجراحية ونظام الإضاءة الإضافي.
يقدم الخبراء الطبيون أنواعًا مختلفة من وحدات العناية المركزة وأسرّة التمريض بالمستشفى والتي تتميز بتصميم جميل حقًا ودائم الاستخدام.
إنها محطات عمل مثالية للغازات الطبية وإمدادات الطاقة ومنصات الأجهزة ورفوف مضخات التسريب ومحطات إخراج الشبكة في المستشفيات.
المنتجات الطبية غير مسموح بها أي مخالفات. نمتلك أفضل مهندس تقني للمشاركة في تصميم المنتج وتقييمه ، وملتزمون بتقديم مستوى عالي الكفاءة من الخدمة لجميع العملاء.
بالإضافة إلى تصنيع المعدات الطبية ، ننخرط أيضًا في البحث وتطوير صناعات التكنولوجيا الحديثة والعالية ، ودعم المؤسسات الطبية لتحسين تأثير التشخيص والعلاج في الأمراض المختلفة.
شاركت Expert Medical في تصنيع المعدات الطبية والخدمات الفنية لأكثر من 17 عامًا ، وتواصل تقديم معلومات السوق في الوقت المناسب والدعم الفني لجميع العملاء ، مع فريق فني محترف لتلبية المتطلبات المختلفة لخدمة ما قبل البيع وما بعد البيع.
يقع Expert Medical في مدينة Qufu ، مقاطعة Shandong ، وهي أكبر مجموعة صناعة المعدات الجراحية في الصين وحتى في العالم. منتجاتنا الرئيسية هي: سلسلة المصابيح الجراحية ، سلسلة الطاولات الجراحية ، القلادة الطبية ، سلسلة الأسرة الطبية إلخ ، أكثر من 200 مواصفة.

نحن نحب ما نفعل
من الأفضل أن تكون متخصصًا جيدًا في عمل واحد بدلاً من أن تكون متخصصًا في العديد من الصناعات.
يدرك فريق تصميم وتصنيع المعدات الطبية لدينا جيدًا احتياجات المرضى والأطباء. لذلك ، نحن على ثقة من أننا خبراء في صناعة المعدات الطبية.
نحن إحدى الشركات الرائدة في تصنيع المعدات الطبية عالية التقنية ، ويتم توزيع المنتجات على نطاق واسع في المستشفيات والعيادات حول العالم وتدعمها شبكة موزعين واسعة النطاق.
تخضع جميع مراحل الإنتاج لمنتجاتنا لرقابة صارمة ، وتخضع كل خطوة لرقابة واختبار الجودة الدقيقة والمهنية. جميع المنتجات لها أداء شبه مثالي في الممارسة الطبية.
بالإضافة إلى معدات التشغيل الجراحية الأساسية وأدوات المستشفى ، فإننا ننتج أيضًا ونوفر معدات التشخيص الطبي والعلاج الرقمي وجهاز الاسترداد وسلسلة المنتجات الأخرى ، ومئات الأصناف والمواصفات لتلبية المتطلبات المختلفة. وقف مزود حلول المعدات الطبية.
بصفتنا شركة متخصصة في تصنيع المعدات الطبية وقفة واحدة ، تمتلك EXPERT MEDICAL سلسلة إمداد متكاملة لتوفير مجموعة كاملة من خدمات المعدات الطبية لغرفة العمليات ومناطق الرعاية الحرجة ومؤسسات الرعاية الأولية وما إلى ذلك ، مع تكنولوجيا الإنتاج الحديثة وضوابط صارمة للتكلفة ، نحن نوفر المنتجات الأكثر فعالية من حيث التكلفة لجميع عملائنا.

من الأسرة الأساسية والأثاث الطبي والعربات إلى معدات غرفة العمليات والاختبارات التشخيصية والعلاج الطبيعي لإعادة التأهيل ، فإن خبرة فريق المعدات الطبية الخبير في إدارة المخاطر والمشاريع تجعلنا المورد المفضل للمستشفيات في أكثر من 70 دولة. سنستمر أيضًا في إفساح المجال الكامل لمزايانا وتوفير معدات طبية احترافية.
محورنا الرئيسي هو الابتكار المستمر. نحن ملتزمون بالتطوير والتنفيذ المستمر لمعايير جديدة لجودة المنتج وكفاءة الخدمة. لقد جمعنا العديد من براءات الاختراع والشهادات الفنية المسجلة. حيث نسعى جاهدين لتقديم أفضل تجربة لعملائنا والمساهمة في تحسين وتحديث أنظمة الرعاية الصحية في جميع أنحاء العالم.
EXPERT MEDICAL ليس فقط منتجات المبيعات ، بل نقدم أيضًا التدريب المناسب على التركيب والتعليم لمساعدة العملاء في أداء أعمال بأعلى المعايير. هناك عنصر أخلاقي في كل هذا ، حيث تدعم شركة EXPERT MEDICAL قيم النزاهة والأمانة ، بينما تتعامل دائمًا مع جميع العملاء كشركاء تعاون ودي.
نحن نتفهم المقصد الأصلي والأسباب التي تجعل العملاء يقومون بتخصيص المعدات الطبية ، حتى نتمكن من توفير أنسب المعدات. لدينا فريق بحث وتطوير محترف لتصميم ومطابقة طلبات العملاء المختلفين حول المعدات الطبية المخصصة وتوفير المنتجات بمواصفات مختلفة وفقًا للطلبات المختلفة لضمان كفاءة العمل بشكل أعلى ، والآن دعنا نعرف طلبات التخصيص الخاصة بك لتزويدك بأكثر المعدات والمنتجات المهنية.
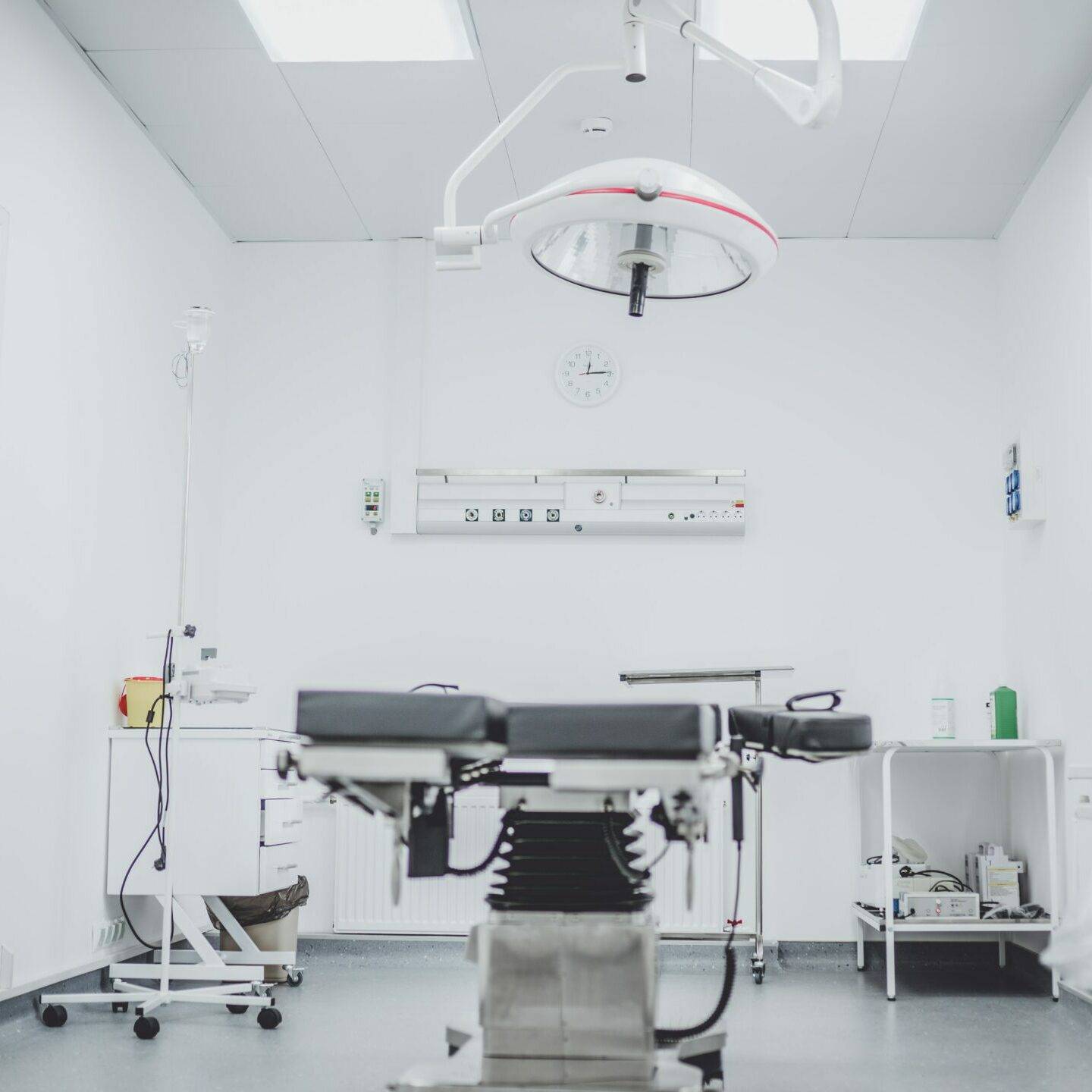
EXPERT MEDICAL مكرس لإنشاء نظام جودة مطمئن خلال عملية تصنيع المعدات الطبية بأكملها. لهذه القيمة الأساسية ، نحن مسؤولون عن كل من عملائنا والصناعة بأكملها.
This guide explores the key factors to consider when choosing...
اقرأ أكثرChoosing the right hospital electrical bed can significantly impact patient...
اقرأ أكثريستكشف هذا الدليل الشامل الجوانب الرئيسية للكهرباء الطبية...
اقرأ أكثر
سلسلة جداول العمليات الطبية الخبيرة تلبي متطلبات الجراحة العامة والقلب والرأس والرقبة وتجويف الصدر وغيرها من العمليات الجراحية.

سلسلة مصابيح التشغيل الطبية الخبيرة تشارك في مختلف مجالات الإضاءة الجراحية ونظام الإضاءة الإضافي.

يقدم الخبراء الطبيون أنواعًا مختلفة من وحدات العناية المركزة وأسرّة التمريض بالمستشفى والتي تتميز بتصميم جميل حقًا ودائم الاستخدام.
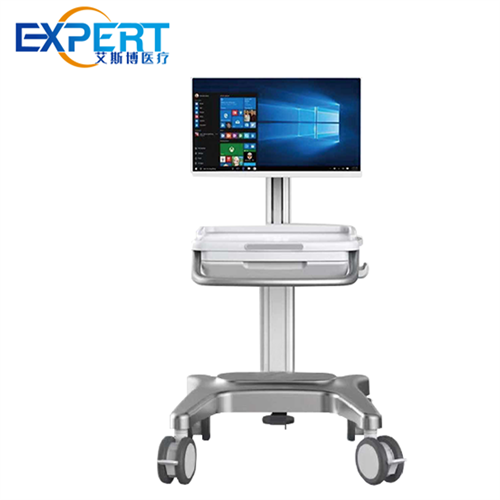
سواء كانت رعاية طبية يومية أو جراحة ، يمكن أن تساعدك عربات الجراحة الطبية الخبيرة في العمل بكفاءة أكبر.
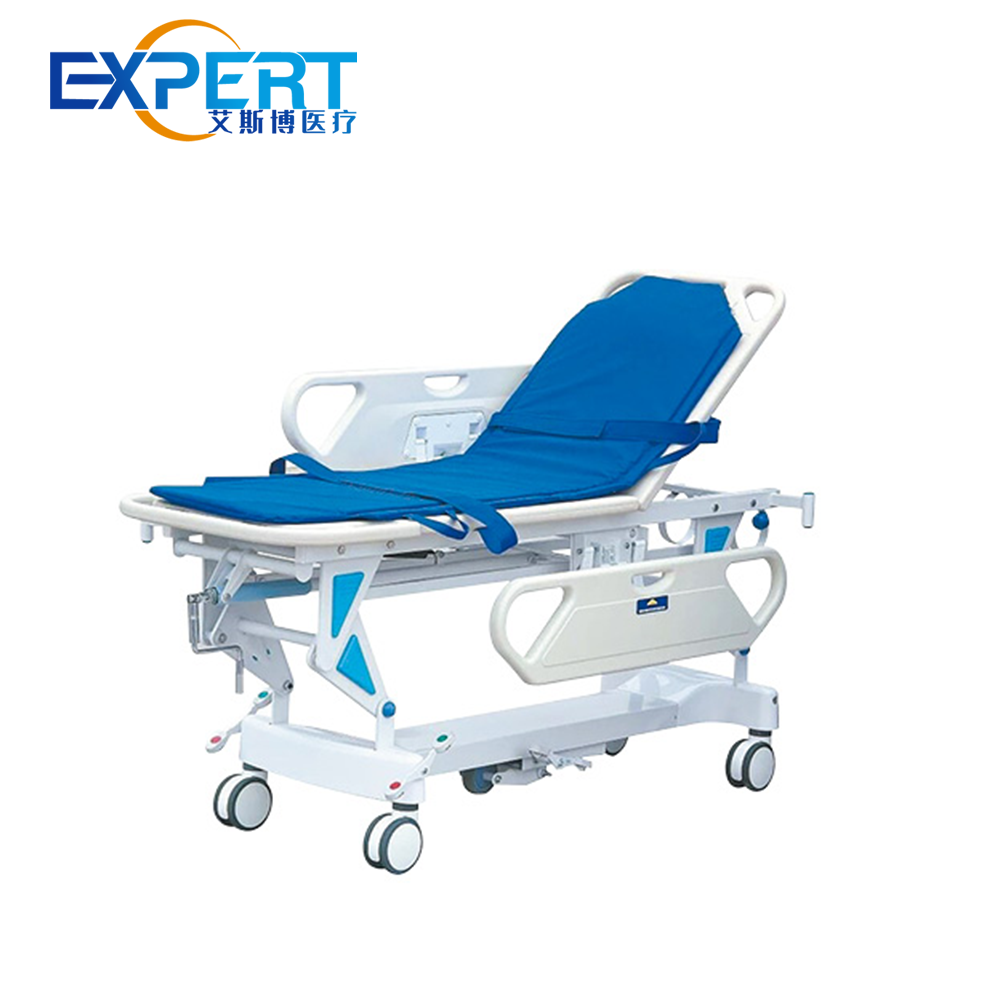
مع مجموعة متنوعة من الهياكل والوظيفية ، توفر نقالات الخبراء دعمًا قويًا في عمليات الإنقاذ في حالات الطوارئ ونقل المرضى.

تعمل الأجهزة الطبية الأخرى الخاصة بـ Expert Medical معًا للمساعدة في بناء نظام طبي كامل.
نحن ملتزمون بتوفير نموذج مبيعات شامل للعملاء ؛ نحن نولي اهتمامًا أكثر أهمية بإرضاء عملائنا.
